Seis parámetros importantes de las baterías de litio
1. Capacidad de la batería
Tabla de contenido
- 1. Capacidad de la batería
- 2. Tensión nominal
- 3. Tensión de terminación de carga
- 4. Voltaje de terminación de descarga
- 5. Resistencia interna de la batería
- 6. Tasa de autodescarga
- 6.1 ¿Cómo afecta la tasa de descarga a la capacidad de la batería?
- 6.2 ¿Cuál es la tasa de descarga de las baterías de bicicletas eléctricas?
- 6.3 ¿Qué se considera una tasa de descarga alta para las baterías de litio?
- 6.4 ¿Cuál es una buena tasa de descarga para las baterías?
- 6.5 ¿Cómo se calcula la tasa C?
Generalmente, la capacidad de la batería está determinada por la cantidad de material activo en la batería, generalmente expresada en miliamperios-hora mAh o Ah. Por ejemplo, se pueden descargar 1000 mAh durante 1 h con una corriente de 1 A.
1.1 Comprensión de la capacidad de la batería: nominal, real y teórica
La capacidad de la batería se puede clasificar en capacidad real, capacidad teórica y capacidad nominal, según diferentes condiciones.
La capacidad que proporciona una batería cuando se descarga a una velocidad de descarga particular a 25 °C hasta su voltaje terminal se define como la capacidad de la batería durante el diseño y la producción. Esto se denomina capacidad nominal para una tasa de descarga RH determinada.
La capacidad de la batería normalmente se mide en AH (amperios-hora). Otro método de medición es en términos de CELL (por unidad de placa) en vatios (W/CELL).
- Para los cálculos de Ah (amperios-hora), se toma la corriente de descarga (corriente constante) I y se multiplica por el tiempo de descarga (en horas) T. Por ejemplo, una batería de 7 AH descargada continuamente a 0,35 A puede durar aproximadamente 20 horas.
- El tiempo de carga estándar es de 15 horas y la corriente de carga es 1/10 de la capacidad de la batería. La carga rápida puede reducir la vida útil de la batería.
La capacidad de la batería se refiere al tamaño de la carga eléctrica almacenada en una batería. La unidad de capacidad de la batería es “mAh”, conocida en chino como miliamperios-hora. Para baterías de mayor capacidad, como las de plomo-ácido, generalmente se utiliza “Ah” (amperios-hora) por conveniencia, donde 1Ah = 1000mAh. Si una batería tiene una capacidad nominal de 1300 mAh y la descarga con una corriente de 130 mA, la batería puede funcionar durante aproximadamente 10 horas (1300 mAh/130 mA = 10 h). Si la corriente de descarga es de 1300 mA, el tiempo de funcionamiento se reduce a aproximadamente 1 hora. Estos cálculos suponen condiciones ideales y el funcionamiento real del dispositivo puede variar según los componentes, como una pantalla LCD o el flash de una cámara digital, lo que puede provocar grandes variaciones en la corriente. Por lo tanto, el tiempo de funcionamiento real de un dispositivo alimentado por batería sólo puede ser aproximado, generalmente basándose en la experiencia del mundo real.
1.2 Unidad de Capacidad
Normalmente, la capacidad de la batería se mide en amperios-hora (Ah) y esto se determina teniendo en cuenta una batería específica. Por ejemplo, la pregunta podría ser: ¿Cuál es la capacidad de la batería de este teléfono inteligente? ¿O cuál es la capacidad de la batería de este scooter eléctrico? Estas consultas son únicas para cada batería. Cuando el voltaje de la batería ya está definido y no se está considerando el voltaje en tiempo real, simplemente indicar los amperios-hora puede representar la capacidad de la batería.
Sin embargo, cuando se trata de baterías de diferentes voltajes, no se puede confiar simplemente en los amperios-hora para indicar la capacidad. Tomemos, por ejemplo, unBatería varonil 12V 20AHy una batería MANLY de 15V 20AH. Aunque ambos tienen 20AH, cuando se alimenta un dispositivo con la misma carga, el dispositivo funcionará bien, pero la duración de la operación será diferente. Por tanto, la capacidad estándar debe medirse en términos de potencia.
Para ilustrarlo mejor, considere un dispositivo que admita 12 V y 24 V. Si funciona con una batería MANLY de 12 V y 20 AH, puede durar una hora. Sin embargo, si dos de estas baterías se conectan en serie, lo que da como resultado 24 V 20 AH, los amperios-hora permanecen sin cambios, pero el tiempo de funcionamiento se duplica. En este contexto, la capacidad debe verse en términos de la energía que la batería puede contener, no solo en amperios-hora.
Potencia (W) = Potencia (P) * Tiempo (T) = Corriente (I) * Voltaje (U) * Tiempo (T).
Este enfoque para discutir la capacidad de la batería es más significativo. Hay que ser realista y objetivo; de lo contrario, podría terminar con la afirmación ilógica de que la batería de un teléfono inteligente tiene una capacidad mayor que la de un scooter eléctrico, lo cual es claramente poco científico.
2. Tensión nominal
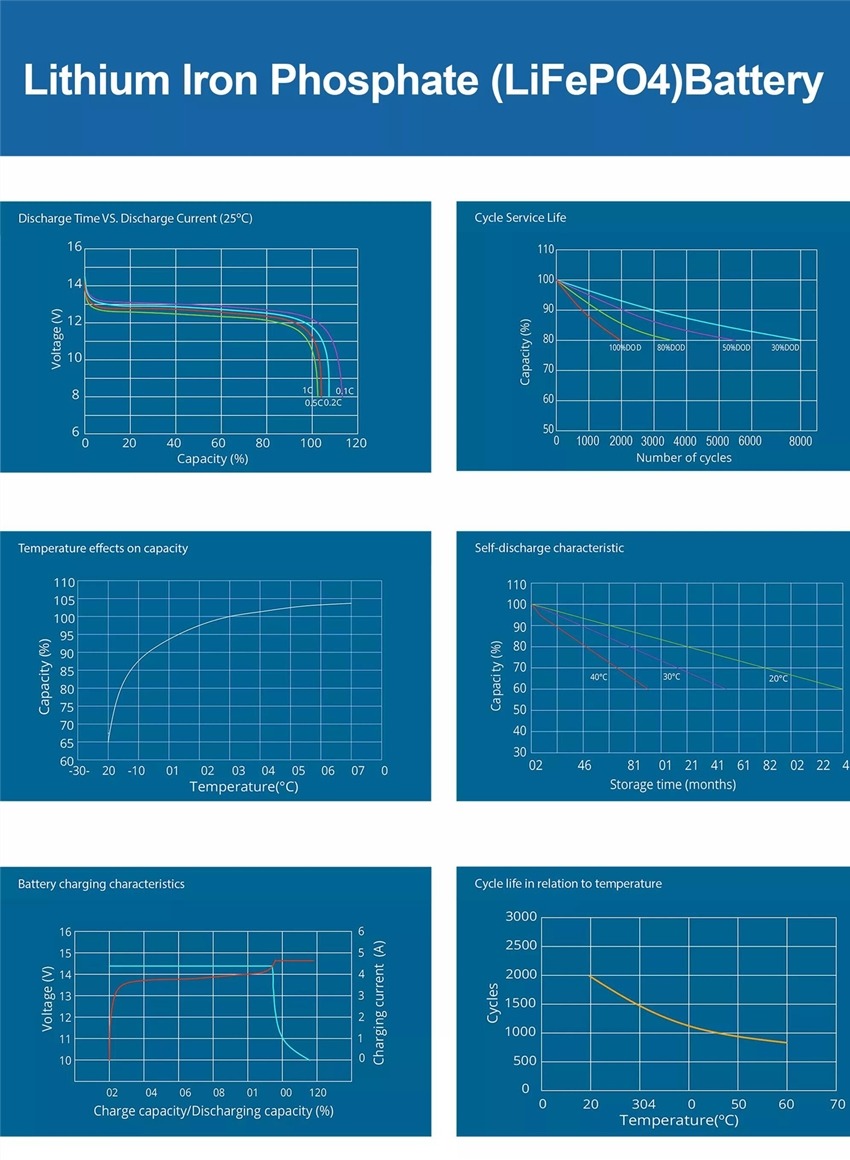
La diferencia de potencial entre los electrodos positivo y negativo de la batería se denomina voltaje nominal de la batería. La tensión nominal está determinada por el potencial del electrodo del material de la placa y la concentración del electrolito interno. El diagrama de descarga de la batería de litio es parabólico, con 4,3 V cayendo a 3,7 V y 3,7 V cayendo a 3,0 V, los cuales cambian rápidamente. Sólo el tiempo de descarga de aproximadamente 3,7 V es el más largo y representa casi 3/4 del tiempo, por lo que el voltaje nominal de la batería de litio se refiere al voltaje que mantiene el tiempo de descarga más largo.
- Batería de litio ternaria
El voltaje nominal de una celda ternaria de litio es de 3,6 V, con un rango de voltaje de funcionamiento entre 2,5 V y 4,2 V. Para un paquete de baterías, multiplica el voltaje por la cantidad de celdas en serie. Por ejemplo, para un paquete de baterías ternarias de litio de la serie 10, el voltaje nominal es de 36 V y el rango de voltaje de funcionamiento abarca de 25 V a 42 V.
- Batería de fosfato de hierro y litio
El voltaje nominal de una celda de fosfato de hierro y litio es de 3,2 V, con un rango de voltaje operativo de 2,0 V a 3,65 V. De manera similar, para el paquete de baterías correspondiente, se multiplica por el número de celdas en serie. Por ejemplo, un paquete de baterías de fosfato de hierro y litio de la serie 15 tiene un voltaje nominal de 48 V, con un rango de voltaje de funcionamiento de 30 V a 54,75 V.
Entonces, ¿cuáles son las implicaciones de un bajo voltaje para las baterías de litio?
Se recomienda almacenar las baterías de litio a largo plazo con aproximadamente el 70% de su carga. Si no se utiliza durante 3 a 6 meses, es recomendable realizar un ciclo de carga y descarga completa. Esto beneficia la vida útil general de la batería.
Si se almacena durante períodos prolongados sin uso y a voltajes muy bajos, los materiales de la batería de litio pueden verse afectados negativamente. Su reactividad química podría deteriorarse, lo que a su vez afecta la vida útil de la batería.
Las baterías de iones de litio funcionan a voltajes que oscilan entre 2,5 V y 4,2 V. Cuando el voltaje cae por debajo de 2,5 V, la descarga de la batería termina y, debido al cierre del circuito de descarga, la pérdida de corriente del circuito de protección interno cae a su nivel más bajo. Sin embargo, en aplicaciones del mundo real, debido a variaciones en los materiales internos, el voltaje de terminación de descarga puede oscilar entre 2,5 V y 3,0 V. Cuando el voltaje excede los 4,2 V, el circuito de carga finaliza para garantizar la seguridad de la batería.
3. Tensión de terminación de carga
Cuando la batería recargable está completamente cargada, el material activo en la placa del electrodo ha alcanzado un estado de saturación y el voltaje de la batería no aumentará cuando la batería continúe cargándose. El voltaje en este momento se llama voltaje de fin de carga. La batería ternaria de litio es de 4,2 V y la batería de fosfato de hierro y litio es de 3,65 V.
4. Voltaje de terminación de descarga
El voltaje de fin de descarga se refiere al voltaje más bajo permitido cuando la batería está descargada. El voltaje de terminación de descarga está relacionado con la tasa de descarga.
5. Resistencia interna de la batería
La resistencia interna de la batería está determinada por la resistencia de la placa del electrodo y la resistencia del flujo de iones. Durante el proceso de carga y descarga, la resistencia del motor de imágenes y de la placa del electrodo no cambia, pero la resistencia del flujo de iones aumentará o disminuirá con la concentración del electrolito y los iones cargados. Y cambio. Cuando el voltaje OCV de una batería de litio disminuye, la impedancia aumentará. Por lo tanto, cuando se carga a baja potencia (menos de 3 V), se debe realizar primero la precarga (carga lenta) para evitar que demasiada corriente provoque una generación excesiva de calor en la batería.
Composición de la resistencia interna de la batería de litio
La resistencia óhmica surge principalmente de los materiales de los electrodos, del electrolito, de la resistencia del separador, así como de la resistencia de contacto de los colectores de corriente y de las conexiones de lengüeta. Está relacionado con el tamaño, la estructura y los métodos de conexión de la batería.
La resistencia a la polarización, que surge instantáneamente cuando se aplica corriente, representa la tendencia acumulativa de varias barreras dentro de la batería que impiden que los iones cargados lleguen a sus destinos. Esta resistencia se puede clasificar además en polarización electroquímica y polarización de concentración.
Actualmente, la destacada batería de litio 18650 tiene una resistencia interna de alrededor de 12 miliohmios, mientras que las típicas oscilan entre 13 y 15 miliohmios. Dado que la impedancia puede afectar el rendimiento de la batería, en términos generales, 50 miliohmios se consideran normales. Entre 50 y 100 miliohmios, la batería sigue funcionando, pero el rendimiento comienza a degradarse. Cuando se superan los 100 miliohmios, es necesario el uso en paralelo y cualquier valor superior a 200 miliohmios es prácticamente inutilizable.
Impactos de la resistencia interna de la batería de litio
Todos los factores que impiden el movimiento de iones de litio y electrones de un polo a otro dentro de una batería de litio contribuyen a su resistencia interna. Idealmente, cuanto menor sea la resistencia interna, mejor. Una mayor resistencia interna conduce a mayores pérdidas térmicas, lo que evita la descarga de corriente elevada. Además, una alta resistencia interna significa que la batería se calienta durante el uso. Las temperaturas elevadas pueden hacer que el voltaje de funcionamiento de descarga de la batería caiga y su duración de descarga se acorte, lo que afecta gravemente el rendimiento y la vida útil de la batería. En casos extremos, esto puede incluso suponer un riesgo de combustión espontánea.
6. Tasa de autodescarga
Se refiere al porcentaje de la capacidad total que se pierde automáticamente cuando la batería no se utiliza durante un período de tiempo. Generalmente, la tasa de autodescarga de las baterías de iones de litio a temperatura ambiente es del 5% al 8%.
6.1 ¿Cómo afecta la tasa de descarga a la capacidad de la batería?
La tasa de descarga afecta directamente la capacidad efectiva de una batería. Específicamente, una tasa de descarga más alta puede disminuir la capacidad disponible, ya que es posible que la batería no pueda mantener su capacidad nominal máxima durante descargas rápidas. Por tanto, al evaluar la capacidad utilizable de una batería, se debe tener en cuenta la tasa de descarga.
6.2 ¿Cuál es la tasa de descarga de las baterías de bicicletas eléctricas?
La tasa de descarga de las baterías de bicicletas eléctricas puede variar según la química y el diseño específicos de la batería. Las bicicletas eléctricas suelen utilizar baterías de iones de litio debido a su alta densidad de energía y rendimiento. Estas baterías generalmente presentan una tasa de descarga que oscila entre 1C y 4C o incluso más. A modo de ejemplo, una batería de 1 Ah con una tasa de descarga de 10 C puede entregar una corriente de descarga continua de 10 amperios, mientras que una tasa de 4 C permitiría una corriente de descarga continua de 40 amperios.
6.3 ¿Qué se considera una tasa de descarga alta para las baterías de litio?
Para las baterías de litio, una tasa de descarga que normalmente se considera "alta" comienza en 1C y más. Sin embargo, es importante tener en cuenta que lo que se considera una tasa de descarga específica alta puede variar según el diseño, la composición química y la aplicación prevista de la batería.
6.4 ¿Cuál es una buena tasa de descarga para las baterías?
Una tasa C óptima para una batería depende de las demandas específicas de su aplicación. Normalmente, se considera favorable una tasa de descarga que facilite la transferencia eficiente de energía sin estresar demasiado la batería. Se recomienda consultar las especificaciones y pautas del fabricante para determinar la mejor tasa de descarga para una batería en particular.
6.5 ¿Cómo se calcula la tasa C?
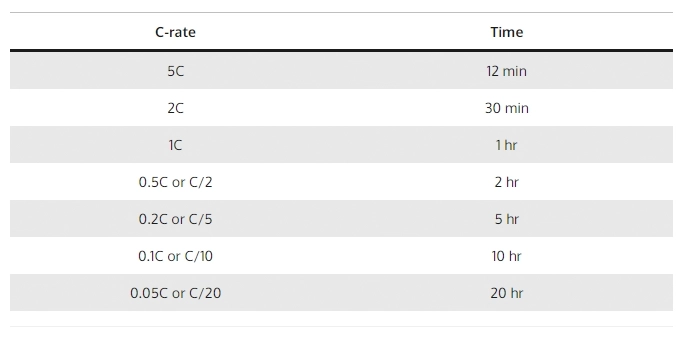
Velocidad C (C) = Corriente de carga o descarga en amperios (A) / Capacidad nominal de la batería (Ah)
Profundicemos en un ejemplo de una batería de litio de 100Ah:
1C representa una corriente de descarga de 100 amperios, lo que significa que la batería puede proporcionar una descarga continua de 100 amperios durante una hora. En términos más simples, puede manejar una corriente de carga de 100 amperios durante 60 minutos.
Si aumentamos la velocidad C a 2C, la corriente de descarga se convierte en 200 amperios. Esto significa que la batería ahora puede proporcionar una corriente de descarga de 200 amperios, pero durante un tiempo reducido. A una velocidad de 2C, la batería puede soportar una corriente de carga de 200 amperios durante 30 minutos o media hora.
Por otro lado, reducir la tasa C a 0,5 C da como resultado una corriente de descarga de 50 amperios. A una velocidad de 0,5 C, la batería puede entregar una corriente de descarga de 50 amperios, prolongando así el período de descarga. En estas condiciones, la batería puede soportar una corriente de carga de 50 amperios durante 2 horas o 120 minutos.
Al evaluar el rendimiento y la capacidad de las baterías de litio, la tasa C se destaca como un factor crucial ya que determina tanto la corriente de descarga disponible como la duración de descarga correspondiente.