Six Important Parameters of Lithium Batteries
1. Battery capacity
Table of Contents
Generally the capacity of the battery is determined by the amount of active material in the battery, usually expressed in milliampere-hour mAh or Ah. For example, 1000 mAh can be discharged for 1 h with a current of 1 A.
1.1 Understanding Battery Capacity: Rated vs. Actual vs. Theoretical
Battery capacity can be categorized into actual capacity, theoretical capacity, and rated capacity, based on different conditions.
The capacity that a battery provides when discharged at a particular discharge rate at 25°C down to its terminal voltage is defined as the battery’s capacity during design and production. This is termed the rated capacity for a given discharge rate RH.
Battery capacity is typically measured in AH (Ampere-hours). Another method of measurement is in terms of CELL (per unit plate) in watts (W/CELL).
- For Ah (Ampere-hours) calculations, you take the discharge current (constant current) I and multiply it by the discharge time (in hours) T. For instance, a 7AH battery discharged continuously at 0.35A can last for approximately 20 hours.
- The standard charging time is 15 hours, and the charging current is 1/10 of the battery’s capacity. Fast charging may reduce the battery’s lifespan.
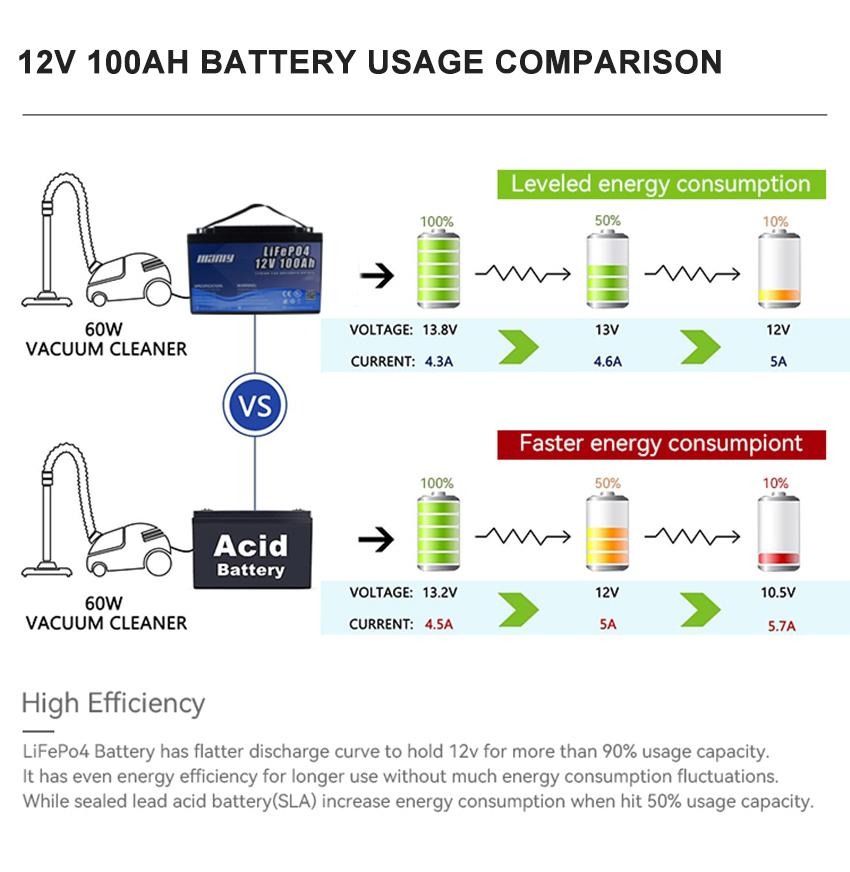
Battery capacity refers to the size of the stored electrical charge in a battery. The unit for battery capacity is “mAh”, known in Chinese as milliampere-hours. For larger capacity batteries, such as lead-acid batteries, “Ah” (Ampere-hours) is generally used for convenience, where 1Ah = 1000mAh. If a battery has a rated capacity of 1300mAh, and you discharge the battery with a current of 130mA, the battery can operate for about 10 hours (1300mAh/130mA = 10h). If the discharge current is 1300mA, then the operational time drops to around 1 hour. These calculations assume ideal conditions, and actual device operation may vary based on components, like an LCD screen or flash in a digital camera, which can cause large variations in current. Hence, the actual operational time for a device powered by the battery can only be approximated, usually based on real-world experience.
1.2 Unit of Capacity
Typically, battery capacity is measured in ampere-hours (Ah), and this is determined for a specific battery in mind. For instance, the question might be: What’s the capacity of this smartphone battery? Or, what’s the capacity of this electric scooter battery? These queries are unique to each battery. When the battery voltage is already defined and one isn’t considering the real-time voltage, merely stating the ampere-hours can represent the battery’s capacity.
However, when dealing with batteries of different voltages, one can’t simply rely on ampere-hours to denote capacity. Take, for instance, a MANLY 12V 20AH battery and a MANLY 15V 20AH battery. Even though both have 20AH, when powering a device with the same load, the device will work just fine, but the duration of operation will differ. Hence, the standard capacity should be measured in terms of power.
To illustrate further, consider a device that can support both 12V and 24V. If powered by a MANLY 12V 20AH battery, it can last for one hour. However, if two such batteries are connected in series, resulting in 24V 20AH, the ampere-hours remain unchanged, but the operational time doubles. In this context, the capacity should be viewed in terms of the power the battery can hold, not just the ampere-hours.
Power (W) = Power (P) * Time (T) = Current (I) * Voltage (U) * Time (T).
This approach to discussing battery capacity is more meaningful. One must be realistic and factual; otherwise, you might end up with the illogical claim that a smartphone battery has a larger capacity than an electric scooter battery, which is clearly unscientific.
2. Nominal voltage
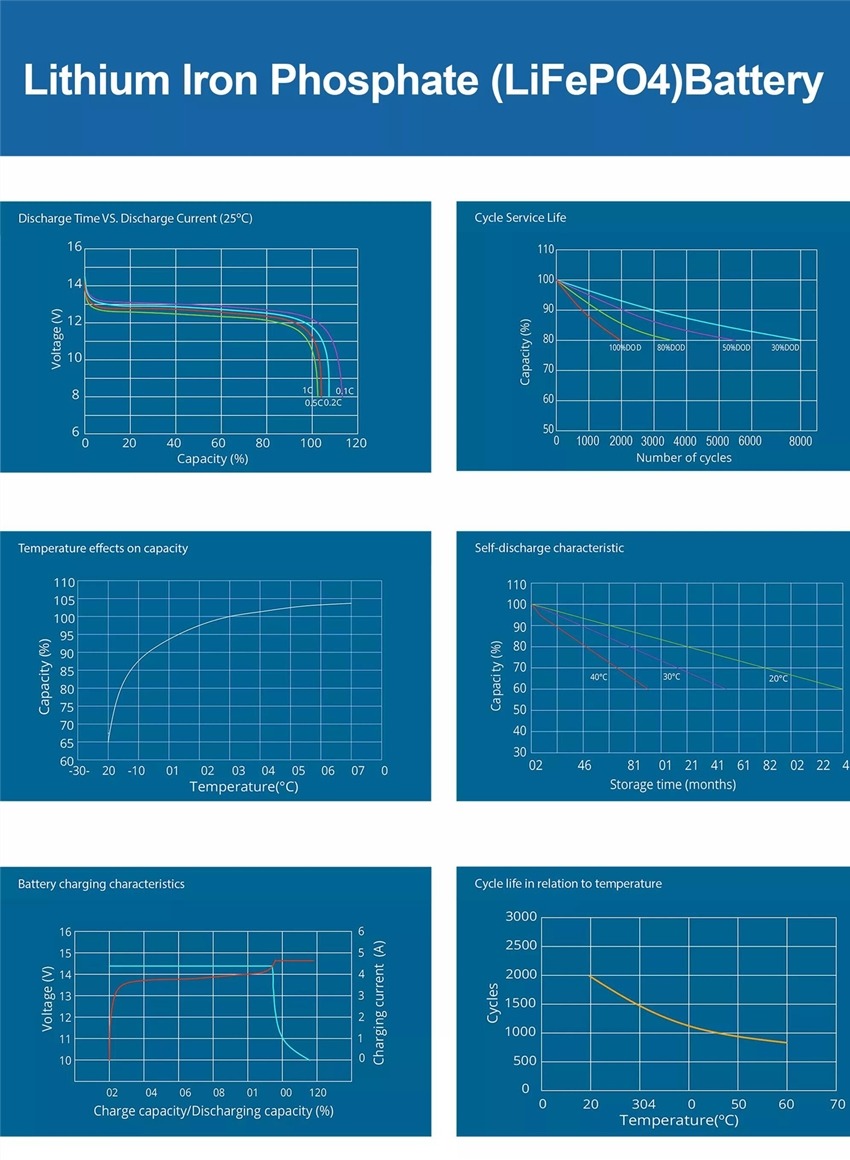
The potential difference between the positive and negative electrodes of the battery is called the nominal voltage of the battery. The nominal voltage is determined by the electrode potential of the plate material and the concentration of the internal electrolyte. The discharge diagram of lithium battery is parabolic, with 4.3V dropping to 3.7V and 3.7V dropping to 3.0V, both of which change rapidly. Only the discharge time of about 3.7V is the longest, accounting for almost 3/4 of the time, so the nominal voltage of the lithium battery refers to the voltage that maintains the longest discharge time.
- Ternary Lithium Battery
The nominal voltage of a ternary lithium cell is 3.6V, with an operating voltage range between 2.5V and 4.2V. For a battery pack, you multiply the voltage by the number of cells in series. For instance, for a 10-series ternary lithium battery pack, the nominal voltage stands at 36V, and the working voltage range spans from 25V to 42V.
- Lithium Iron Phosphate Battery
The nominal voltage of a lithium iron phosphate cell is 3.2V, with an operational voltage range of 2.0V to 3.65V. Similarly, for the corresponding battery pack, you multiply by the number of cells in series. For example, a 15-series lithium iron phosphate battery pack has a nominal voltage of 48V, with a working voltage range of 30V to 54.75V.
So, what are the implications of a low voltage for lithium batteries?
It’s recommended that lithium batteries be stored long-term with about 70% of their charge. If not in use for 3 to 6 months, it’s advisable to cycle through one full charge and discharge. This benefits the overall lifespan of the battery pack.
If stored for extended periods without use and at very low voltages, the materials in the lithium battery can be adversely affected. Their chemical reactivity might deteriorate, which in turn impacts the battery pack’s lifespan.
Lithium-ion batteries operate at voltages ranging from 2.5V to 4.2V. When the voltage drops below 2.5V, the battery discharge terminates, and due to the closing of the discharge circuit, the current loss of the internal protection circuit drops to its lowest. However, in real-world applications, due to variations in internal materials, the discharge termination voltage can range from 2.5V to 3.0V. When the voltage exceeds 4.2V, the charging circuit is terminated to ensure the battery’s safety.
3. Charge termination voltage
When the rechargeable battery is fully charged, the active material on the electrode plate has reached a saturated state, and the battery voltage will not rise when the battery continues to be charged. The voltage at this time is called the end-of-charge voltage. The ternary lithium battery is 4.2V, and the lithium iron phosphate battery is 3.65V.
4. Discharge termination voltage
The end-of-discharge voltage refers to the lowest voltage allowed when the battery is discharged. The discharge termination voltage is related to the discharge rate.
5. Internal resistance of the battery
The internal resistance of the battery is determined by the resistance of the electrode plate and the resistance of the ion flow. During the charging and discharging process, the resistance of the image engine and the electrode plate is unchanged, but the resistance of the ion flow will increase or decrease with the concentration of the electrolyte and the charged ions. And change. When the OCV voltage of a lithium battery decreases, the impedance will increase. Therefore, when charging at low power (less than 3V), pre-charge (trickle charging) must be carried out first to prevent too much current from causing excessive heat generation of the battery.
Composition of Lithium Battery Internal Resistance
Ohmic resistance mainly arises from the electrode materials, electrolyte, separator resistance, as well as the contact resistance of current collectors and tab connections. It’s related to the battery’s size, structure, and connection methods.
Polarization resistance, which emerges instantly when current is applied, represents the cumulative tendency of various barriers inside the battery preventing charged ions from reaching their destinations. This resistance can be further categorized into electrochemical polarization and concentration polarization.
Currently, the standout 18650 lithium battery has an internal resistance of around 12 milliohms, while typical ones hover between 13 to 15 milliohms. Given that impedance can affect the battery’s performance, generally speaking, 50 milliohms is deemed normal. Between 50 to 100 milliohms, the battery remains functional, but performance starts to degrade. When exceeding 100 milliohms, parallel use is necessary, and anything above 200 milliohms is virtually unusable.
Impacts of Lithium Battery Internal Resistance
All factors that impede the movement of lithium ions and electrons from one pole to another within the lithium battery contribute to its internal resistance. Ideally, the lower the internal resistance, the better. A higher internal resistance leads to increased thermal losses, preventing high current discharge. Moreover, a high internal resistance means the battery heats up during use. Elevated temperatures can cause the battery’s discharge operating voltage to drop and its discharge duration to shorten, severely affecting battery performance and lifespan. In extreme cases, this can even pose a risk of spontaneous combustion.
6. Self-discharge rate
It refers to the percentage of the total capacity that is automatically lost when the battery is not in use for a period of time. Generally, the self-discharge rate of lithium-ion batteries at room temperature is 5%-8%.
6.1 How Does the Discharge Rate Affect Battery Capacity?
The discharge rate directly impacts a battery’s effective capacity. Specifically, a higher discharge rate can decrease the available capacity, as the battery might not be able to maintain its maximum rated capacity during rapid discharges. Thus, when evaluating a battery’s usable capacity, the discharge rate must be taken into account.
6.2 What is the Discharge Rate of Electric Bicycle Batteries?
The discharge rate of electric bicycle batteries can vary based on the specific battery chemistry and design. Electric bikes typically employ lithium-ion batteries due to their high energy density and performance. These batteries generally demonstrate a discharge rate ranging from 1C to 4C or even higher. To illustrate, a 1Ah battery with a 10C discharge rate can deliver a continuous discharge current of 10 amps, whereas a 4C rate would allow for a continuous discharge current of 40 amps.
6.3 What is Considered a High Discharge Rate for Lithium Batteries?
For lithium batteries, a discharge rate typically considered “high” starts at 1C and above. However, it’s important to note that what’s deemed as a high specific discharge rate may vary based on the battery’s design, chemical composition, and intended application.
6.4 What is a Good Discharge Rate for Batteries?
An optimal C-rate for a battery hinges on the specific demands of its application. Typically, a discharge rate that facilitates efficient power transfer without overly stressing the battery is regarded as favorable. It’s recommended to consult the manufacturer’s specifications and guidelines to ascertain the best discharge rate for a particular battery.
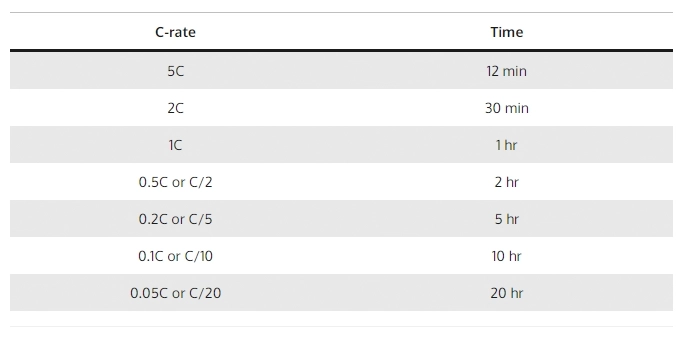
6.5 How Do You Calculate the C-rate?
C-rate (C) = Charging or discharging current in amperes (A) / Battery’s rated capacity (Ah)
Let’s delve into an example involving a 100Ah lithium battery:
1C represents a discharge current of 100 amps, meaning the battery can provide a continuous discharge of 100 amps for one hour. In simpler terms, it can handle a load current of 100 amps for 60 minutes.
If we boost the C-rate to 2C, the discharge current becomes 200 amps. This signifies that the battery can now furnish a discharge current of 200 amps, but for a reduced duration. At a 2C rate, the battery can sustain a load current of 200 amps for 30 minutes or half an hour.
On the other hand, reducing the C-rate to 0.5C results in a discharge current of 50 amps. At a 0.5C rate, the battery can deliver a discharge current of 50 amps, thereby prolonging the discharge period. Under these conditions, the battery can support a load current of 50 amps for 2 hours or 120 minutes.
When assessing the performance and capacity of lithium batteries, the C-rate stands out as a crucial factor since it determines both the available discharge current and the corresponding discharge duration.