What is a lithium battery made of?
Table of Contents
- Among the positive electrode materials, the most commonly used materials are lithium cobaltate, lithium manganate, lithium iron phosphate and ternary materials (polymers of nickel, cobalt and manganese). The positive electrode material accounts for a large proportion (the mass ratio of the positive and negative electrode materials is 3:1~4:1), because the performance of the positive electrode material directly affects the performance of the lithium-ion battery, and its cost also directly determines the cost of the battery.
- Among the negative electrode materials, natural graphite and artificial graphite are currently the main negative electrode materials. Anode materials being explored include nitrides, polyaspartic acid, tin-based oxides, tin alloys, nano-anode materials, and other intermetallic compounds. As one of the four major materials of lithium batteries, negative electrode materials play an important role in improving battery capacity and cycle performance, and are at the core of the middle reaches of the lithium battery industry.
- The market-oriented diaphragm materials are mainly polyolefin diaphragms, which are mainly made of polyethylene and polypropylene. In the structure of the lithium battery separator, the separator is one of the key internal components. The performance of the separator determines the interface structure and internal resistance of the battery, which directly affects the capacity, cycle and safety performance of the battery. A separator with excellent performance plays an important role in improving the overall performance of the battery.
- The electrolyte is generally made of high-purity organic solvents, electrolyte lithium salts, necessary additives and other raw materials in a certain proportion under certain conditions. The electrolyte plays the role of conducting ions between the positive and negative electrodes of the lithium battery, which is the guarantee of high voltage and high specific energy of the lithium ion battery.
- Battery casing: divided into steel casing, aluminum casing, nickel-plated iron casing (for cylindrical batteries), aluminum-plastic film (soft packaging), etc., as well as the battery cap, which is also the positive and negative terminals of the battery.
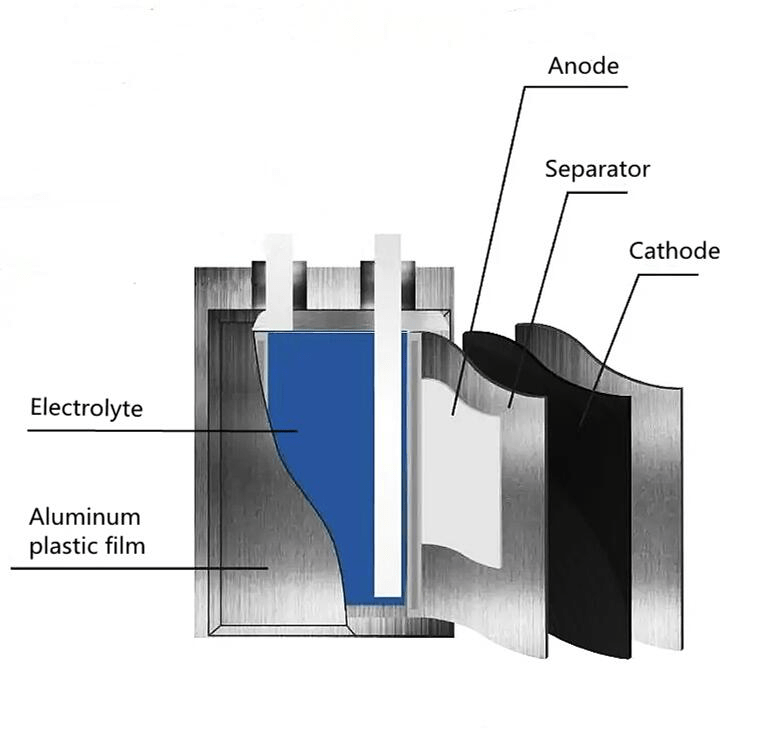
- The principle of Battery work
- When the battery is charged, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte. The carbon structure of the negative electrode has many pores, and the lithium ions reaching the negative electrode are embedded in the micropores of the carbon layer. The more lithium ions are embedded, the higher the charging capacity will be.When the battery is discharged, the lithium ions embedded in the carbon layer of the negative electrode come out and return to the positive electrode. The more lithium ions that go back to the positive electrode, the higher the discharge capacity. Generally speaking, the discharge capacity refers to the discharge capacity.During the charging and discharging process of a lithium battery, lithium ions are in a state of movement from the positive electrode to the negative electrode. If the image of a lithium battery is compared to a rocking chair, the two ends of the rocking chair are the positive and negative electrodes of the battery, and lithium ions are like athletes, running back and forth between the two ends of the rocking chair. So lithium batteries are also called rocking chair batteries.
 MANLY 10KWh Powerwall Battery For Home Energy
MANLY 10KWh Powerwall Battery For Home Energy





















