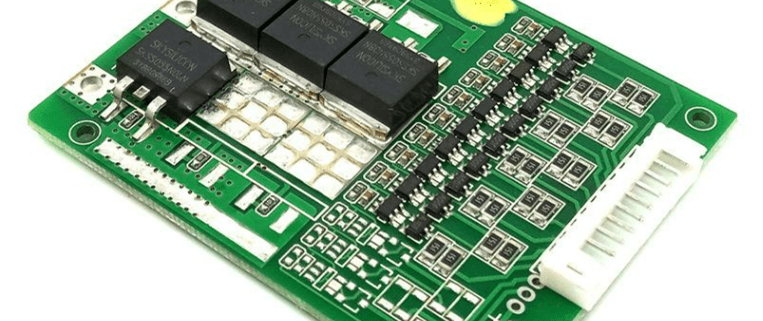
What Type of Battery is a Marine Battery? A Detailed Breakdown
Table of Contents
- What Type of Battery is a Marine Battery? A Detailed Breakdown
- What is a Marine Battery?
- Different Types of Marine Batteries and Their Features
- The Best Choice: LiFePO4 Batteries for Marine Use
- Why LiFePO4 Batteries Are the Best Marine Batteries?
- Conclusion
- FAQ
- Hot Search
- 2023 Lithium Ion vs Lead Acid: A Detailed Comparison
- What Are Lithium Ion Batteries?
- What Is Lead Acid Battery?
- Lithium Ion vs Lead Acid Battery: 10 Key Differences
- Lithium Ion vs Lead Acid: Which Lasts Longer?
- Lithium Ion vs Lead Acid: Prolonging Your Lithium Ion Battery’s Health
- 6 Primary Types of Lithium Ion Batteries
- LiFePO4 Batteries: Leading the Charge in Lithium-Ion Technology
- Lithium Iron Phosphate Battery Vs Lead acid
- MANLY LiFePO4 Battery vs Other Brands LiFePO4 Battery
- Unlocking the Potential of Lithium Batteries Across Industries
- Six Important Parameters of Lithium Batteries
- 1. Battery capacity
- 2. Nominal voltage
- 3. Charge termination voltage
- 4. Discharge termination voltage
- 5. Internal resistance of the battery
- 6. Self-discharge rate
- Lithium battery PCM principle
Maintaining a consistent and dependable boating experience depends on choosing the right marine battery. Whether you use a trolling motor, start your engine, or run electronics, the appropriate battery type will make all the difference. The several choices for batteries make many sailors find it difficult to choose the right one.
This article will break apart lead-acid, AGM, gel, and LiFePO4 batteries as well as other types of marine batteries. You will learn every type in great depth, including its benefits, drawbacks, and optimal uses. By the conclusion, you will understand the reason LiFePO4 batteries are the best fit for marine applications.
What is a Marine Battery?
Designed especially to withstand adverse sea conditions, a marine battery is Unlike automobile batteries, which only provide short bursts of electricity to start an engine, marine batteries must deliver constant power for lengthy durations while tolerating vibrations, dampness, and temperature variations.
- Usually driven by its intended use, marine batteries are categorized:
- The starting battery runs the boat motor. It gives a brief, sharp boost but lacks long-term vitality.
- Deep-cycle batteries run constantly for lights, fish finders, and trolling motors among other equipment.
- A dual-purpose battery sometimes performs worse than dedicated beginning and deep cycle batteries, even if it aims to combine two uses.
These classification indicate the use of a marine battery, although the most important factor influencing battery performance is battery chemistry. Chemistry of a battery determines its general performance, longevity, efficiency, and maintenance requirements.
Different Types of Marine Batteries and Their Features
The several kinds of marine batteries are listed below, together with their characteristics:
Lead-Acid Marine Batteries
Lead-acid batteries have been used in marine conditions as they have been rather competitively priced and widely available for decades. These batteries chemically react with lead plates and sulphuric acid to generate power.
Flooded Lead-Acid (FLA) Batteries
Flooded lead-acid batteries are the most classic form. They demand consistent maintenance, including topped off distilled water. They are heavy, require ventilation, have a shorter lifetime than other battery types even if they are economically priced.
Sealed Lead-Acid Batteries
Lead-acid batteries sealed eliminate maintenance needs and are spill-proof. Two most widely used variants that improve performance over typical flooded lead-acid batteries are AGM (absorbed glass mat) batteries and gel batteries.
Advantages of Lead-Acid Marine Batteries
Lead-acid batteries are reasonably cheap and rather numerous. Their great starting current makes them worth using for engine cranking.
Disadvantages of Lead-Acid Marine Batteries
Lead-acid batteries need regular maintenance, weigh a lot, and charge slowly. Usually running for two to four years, their lifetime is short; they break down upon discharge below 50% capacity.
AGM (Absorbed Glass Mat) Marine Batteries
AGM batteries are spill-proof and maintenance-free by absorbing the electrolyte using fiberglass mats, therefore improving traditional lead-acid designs.
How do AGM Batteries Work?
By immobilising the electrolyte inside the glass mat, AGM batteries reduce the possibility of leaking and hence maximise power efficiency. Since this structure lets for better resistance to vibrations and faster charging periods, AGM batteries are a more durable replacement for flooded lead-acid batteries.
Advantages of AGM Marine Batteries
Zero maintenance and faster charging than other lead-acid models describe AGM batteries. Their lifetime is between three and six years, and they have more resistance to deep discharges.
Disadvantages of AGM Marine Batteries
AGM batteries remain heavier than lithium alternatives even with their superior performance. On a tight budget, they also appeal less to boaters since they cost more than flooded lead-acid batteries.
Gel Marine Batteries
Gel batteries use liquid electrolytes from a silica-based gel. Less leaks and greater deep discharge performance are made possible by this chemical composition.
How do Gel Batteries Work?
The gel-based electrolyte lowers chemical reactions, therefore reducing the possibility of overcharging or deep discharge. Applications needing constant, long-term power will find ideal use for these batteries.
Advantages of Gel Marine Batteries
Gel batteries resist high temperatures, maintain a longer lifetime than flooded lead-acid batteries, and require no maintenance.
Disadvantages of Gel Marine Batteries
The slow charging rate of gel batteries is its biggest drawback. Furthermore expensive than AGM and flooded lead-acid batteries are they are. Less forgiving than other battery kinds, overcharging can damage a battery permanently.
The Best Choice: LiFePO4 Batteries for Marine Use
The most intelligent and powerful marine batteries are LiFePO4 (Lithium Iron Phosphate). Unlike lead-acid batteries, which rely on chemical interactions between lead and acid, they offer constant and dependable power by means of lithium-ion technology.
Why LiFePO4 Batteries are Superior?
Among their most important benefits are LiFePO4 batteries’ extended lifespan. Lead-acid batteries run two to six years; LiFePO4 batteries can run ten years or more. Since they weigh up to 70% less than lead-acid batteries and are more easily handled, they also greatly improve boat performance.
Charging time offers still another major advantage. Five times faster charging of LiFePO4 batteries than with lead-acid batteries Unlike lead-acid batteries, which have to be kept above 50% charge, they also enable total discharges free from battery damage.
Are There Any Drawbacks to LiFePO4 Batteries?
The sole negative of LiFePO4 batteries is their starting cost. For seasoned sailors, however, they are the most affordable choice because of their long lifetime, fast charge times, and low maintenance needs.
Why LiFePO4 Batteries Are the Best Marine Batteries?
LiFePO4 batteries have long-term benefits above all other battery models, even if their initial expense makes many sailors unwilling to switch. Boats carrying LiFePO4 batteries had 30% longer running lifetime on electronic systems and trolling motors, according a study by marine engineers.
Savings in weight also improve performance. LiFePO4 technology lets a boat greatly reduce the weight of its battery, therefore enhancing fuel economy and general maneuverability. Boaters looking for dependability, fast charging, and long-lasting performance would best invest in LiFePO4 batteries.
Conclusion
The perfect sailing voyage depends on selecting the right marine battery. Though lead-acid, AGM, and gel batteries are still somewhat popular, none give the efficiency, lifetime, and long-term savings LiFePO4 batteries offer.
LiFePO4 batteries from MANLY Battery provide the finest answer if you want a dependable and high-performance marine battery. Their innovative lithium technology guarantees exceptional energy economy, therefore assuring that your boat keeps running without the trouble of regular replacements.
For premium-quality marine batteries, visit MANLY Battery today and experience the next generation of marine power solutions.
FAQ
1. Is a deep cycle battery the same as a marine battery?
Let’s clarify: a deep cycle battery and a marine battery aren’t the same, although they have similarities. Here’s why:
A deep cycle battery provides a steady power flow over an extended period. It’s designed for applications that need consistent energy, like golf carts, RVs, or renewable energy systems. These batteries are built to handle repeated discharges and recharges without losing performance.
On the other hand, a marine battery is specifically made for boats. However, marine batteries come in two types: starting and deep cycle. A starting battery delivers a quick surge of power to start the engine, while a deep cycle marine battery powers your boat’s electronics and other devices.
So, while some marine batteries are deep cycle, not all deep cycle batteries work as marine batteries. If you’re choosing a battery for your boat, ensure it’s designed for marine use to get the most reliable power.
2. What are the three types of marine batteries?
Marine batteries come in three main types, each serving a different purpose. Understanding their roles helps you pick the right one for your boat:
- Starting Batteries: Starting batteries provide a quick burst of power to start your engine. They’re all about delivering a high-energy jolt for a short time. These batteries are not designed to power other devices, so they focus only on getting your engine running.
- Deep Cycle Batteries: Need power for your boat’s electronics, trolling motor, or other accessories? Deep cycle batteries are your answer. They deliver steady, long-lasting energy and can discharge and recharge multiple times without losing capacity. Perfect for extended trips or when you’re off the grid!
- Dual Purpose Batteries: As the name suggests, dual purpose batteries combine the functions of both starting and deep cycle batteries. They give you enough power to start your engine and supply energy to your accessories. They offer versatility but may not provide as much power as a dedicated starting or deep cycle battery.
Hot Search
Marine Lithium Battery Battery Manufacturer
Hello
What Are Lithium Ion Batteries?
Table of Contents
- What Are Lithium Ion Batteries?
- What Is Lead Acid Battery?
- Lithium Ion vs Lead Acid Battery: 10 Key Differences
- Lithium Ion vs Lead Acid: Which Lasts Longer?
- Lithium Ion vs Lead Acid: Prolonging Your Lithium Ion Battery’s Health
- 6 Primary Types of Lithium Ion Batteries
- LiFePO4 Batteries: Leading the Charge in Lithium-Ion Technology
- Lithium Iron Phosphate Battery Vs Lead acid
- MANLY LiFePO4 Battery vs Other Brands LiFePO4 Battery
- Unlocking the Potential of Lithium Batteries Across Industries
Lithium-ion batteries are currently the most widely used type of rechargeable batteries. They are the power source behind everyday devices like smartphones, laptops, electric vehicles, and much more.
1. The Widespread Presence of Lithium-Ion Batteries
These batteries are an integral part of our daily lives. They are found in numerous gadgets and tools, including cell phones, tablets, laptops, smartwatches, portable chargers, emergency power sources, electric shavers, electric bicycles and cars, public transport vehicles, sightseeing carts, drones, and various electric tools.
2. How Do Lithium Ion Batteries Work?
Lithium ion batteries function by utilizing lithium ions to store energy. This process involves creating a voltage difference between the battery’s positive and negative sides. The battery contains a special part called a separator, which keeps the two sides apart. This separator allows lithium ions to move across it but prevents the flow of electrons.
- Charging and Discharging Process: When you charge a lithium-ion battery, the lithium ions travel from the positive side to the negative side through the separator. Conversely, when the battery is in use (discharging), the ions move back to the positive side. This back and forth movement of ions is what generates the battery’s voltage.
- Powering Your Devices: The voltage created by the lithium-ion battery is used to power electronic devices. When a device is connected to the battery, it directs the electrons, which were blocked by the separator, to flow through the device, providing it with the necessary power.
What Is Lead Acid Battery?
Lead acid batteries are a type of rechargeable battery that harnesses the chemical interaction between lead and sulfuric acid to generate electricity. They’re commonly used in various applications due to their reliability and rechargeability. The core of these batteries is the lead submerged in sulfuric acid, which facilitates a controlled chemical reaction essential for power generation and storage.
1. How Lead Acid Batteries Function
In a lead acid battery, the electrodes are primarily composed of lead and its oxides, while the electrolyte is a sulfuric acid solution. When discharging, the positive electrode is mainly lead dioxide, and the negative electrode is lead. During charging, both electrodes become primarily lead sulfate. A single cell typically has a nominal voltage of 2.0V, can discharge to 1.5V, and charge up to 2.4V. For practical use, six single cells are often connected in series to create a standard 12V battery, with other configurations like 24V, 36V, and 48V also available.
2. Applications of Lead Acid Batteries
Lead acid batteries are versatile and find use in various sectors:
Standby Power Sources
- Telecommunications
- Solar Energy Systems
- Electronic Switch Systems
- Communication Equipment: Base Stations, PBX, CATV, WLL, ONU, STB, Cordless Phones
- Backup Power: UPS, ECR, Computer Backup Systems, Sequence, ETC
- Emergency Equipment: Emergency Lights, Fire and Burglar Alarms, Fire Doors
Primary Power Sources
- Communication Devices: Transceivers
- Power Control Vehicles: Collection Vehicles, Automated Transport Vehicles, Electric Wheelchairs, Cleaning Robots, Electric Cars
- Mechanical Tool Starters: Lawnmowers, Hedge Trimmers, Cordless Drills, Electric Screwdrivers, Electric Snow Sleds
- Industrial Equipment/Instruments
- Photography: Flashlights, VTR/VCR, Movie Lights
- Other Portable Devices
Lithium Ion vs Lead Acid Battery: 10 Key Differences
1. Differences in Material Composition
Both lithium ion and lead acid batteries operate on similar principles, but the materials they use differ significantly. Lead acid batteries employ lead as the anode and lead oxide as the cathode, with sulfuric acid serving as the electrolyte. In contrast, lithium ion batteries use carbon for the anode and lithium oxide for the cathode, with lithium salt as the electrolyte. The flow of ions between the anode and cathode through the electrolyte is what generates power in both types, reversing during charging.
2. Cost Considerations
Initially, lead acid batteries are more affordable and easier to install than lithium ion ones. However, the price of a lithium ion battery can be twice that of a lead acid battery for the same energy capacity. Despite this, lithium ion batteries offer a longer lifespan, making them more cost-effective for long term applications compared to lead acid batteries.
3. Comparing Battery Capacities
Battery capacity reflects the amount of energy a battery can store per unit volume. Lithium ion batteries boast a higher capacity than lead acid batteries, indicating a greater amount of active material within.
4. Energy Density and Specific Energy
Energy density is crucial in selecting the right battery for specific needs, showing the relationship between a battery’s capacity and its weight. Lithium-ion batteries exhibit higher specific energy compared to lead-acid batteries, making them the preferred choice in electric vehicle (EV) applications.
5. Weight and Size
Thanks to their higher energy density and capacity, lithium-ion batteries are lighter and more compact than lead-acid batteries of the same capacity.
6. Depth of Discharge (DoD) Comparison
DoD measures how much of a fully charged battery can be used without needing a recharge. Lead-acid batteries typically have a DoD of 50%, meaning only half the battery’s capacity should be used before recharging. On the other hand, lithium-ion batteries offer a higher DoD of 80%, allowing for extended use. Modern lithium-ion batteries even reach 100% DoD, showcasing their efficiency and endurance.
7. Durability and Longevity
Lead-acid batteries typically have a lifespan of up to two years with proper maintenance, including recharging after 50% usage. Over-draining can limit their life to just one year. In contrast, lithium-ion batteries boast a remarkable durability of up to 10 years, enduring up to 10,000 cycles.
8. Cycle Life
The cycle life of a battery indicates the number of complete charge and discharge cycles it can handle. Lithium-ion batteries often sustain around 5,000 cycles without significant performance loss, even when fully discharged. Lead-acid batteries, however, generally last between 300 to 500 cycles, with full discharge adversely impacting their cycle life.
9. Charging Speed
Charging speed is a key differentiator between these two types. Lithium-ion batteries can charge much faster than lead-acid batteries, making them preferable for applications needing quick recharge, like electric vehicles (EVs).
10. Safety Considerations
Both battery types pose safety risks if mishandled, especially when overcharged. Lead-acid batteries contain corrosive sulfuric acid and can produce explosive gases. Lithium-ion batteries are at risk of thermal runaway, which can also lead to explosions. Manufacturers like CATL and Panasonic Corporation are key players in creating safer battery systems. The battery’s application often dictates the choice between lithium-ion and lead-acid batteries.
Lithium Ion vs Lead Acid: Which Lasts Longer?
1. Understanding Battery Lifespan and Efficiency
When it comes to longevity, lithium ion (Li-ion) batteries generally have a longer life than lead acid batteries. This is mainly due to their higher life cycle numbers, meaning they don’t need replacing as often. This not only cuts down on replacement costs but also aligns with eco-friendly recycling practices. Additionally, Li-ion batteries are tougher, performing more effectively in demanding environments.
- Efficiency Matters: In the lithium ion vs lead acid comparison, efficiency plays a crucial role. Efficiency refers to the percentage of the energy stored in the battery that can be effectively utilized. Li-ion batteries are typically at least 95% efficient, significantly outperforming lead acid batteries, which have efficiencies around 80-85%.
- Battery Discharge Curve: The discharge curve is crucial as it influences how quickly a battery can charge and its effective capacity – the actual amount of energy a battery can store. Li-ion batteries have a superior discharge curve, maintaining their voltage until almost fully depleted, unlike lead acid batteries, which experience a significant voltage drop during discharge.
2. Usage Patterns: A Key Differentiator
- Lithium ion Batteries: Li-ion batteries are known for their speedy charging times, making them ideal for extended use across multiple work shifts. They lack a memory effect, allowing for partial charging without reducing their overall lifespan. A typical usage pattern includes 8 hours of operation, a swift 1-hour recharge, followed by another 8 hours of use. This cycle enables continuous usage over a 24-hour period, with only brief pauses for charging.
- Lead Acid Batteries: In contrast, lead acid batteries generate substantial heat while charging, necessitating a cooldown period. Their typical usage cycle includes 8 hours of operation, followed by 8 hours of charging and an equal period of rest. This pattern limits their use to one shift per day, requiring additional batteries for multi-shift operations. They also need well-ventilated areas for charging to prevent the buildup of hazardous gases.
Lithium Ion vs Lead Acid: Prolonging Your Lithium Ion Battery’s Health
1. Introduction to Lithium Ion Battery Care
Maximizing the lifespan of lithium ion batteries is crucial for ensuring long-term efficiency and performance. Implementing strategies such as partial discharge cycles, avoiding full discharges, and managing charging temperatures can significantly impact their durability. It’s important to note that while lead acid batteries should not be discharged beyond 50%, lithium ion batteries can handle deeper discharge cycles without adverse effects.
2. Key Techniques for Prolonging Battery Life
- Optimal Discharge Cycles: Utilize only 20-30% of the battery’s capacity before recharging. Avoid keeping the battery fully charged or fully discharged for extended periods, as both extremes can shorten its lifespan.
- Temperature Management During Charging: Charging lithium ion batteries in extreme temperatures, particularly below freezing, can reduce their longevity. Ensure the charging environment is temperature controlled to extend battery life.
- Proper Charging Practices: Use the correct charger type for your lithium ion battery to ensure it charges efficiently and safely. Lithium ion batteries require more specific charging regimes compared to lead acid batteries.
- Benefits of Lithium Ion Over Lead Acid: Lithium ion batteries offer a range of advantages over lead acid batteries, including improved performance in challenging environments and overall cost-effectiveness over their lifespan.
3. Detailed Tips for Optimal Battery Usage
- Lowering Discharge Rates: Reducing the C rate during discharge helps maintain the battery’s capacity and cycle life. Avoid high discharge rates to prevent increased internal resistance and premature aging.
- Temperature Considerations: The operating temperature significantly impacts a battery’s power consumption and efficiency. Manage temperatures effectively to enhance lithium ion battery performance.
- Partial Depth of Discharge (DoD): Favor partial discharges over full cycles. A shallower DoD results in a significantly higher number of battery cycles, thereby extending the battery’s lifespan.
- Balancing Multiple Cells: If your battery pack has more than one cell, periodic balancing is necessary to ensure even usage and prevent voltage loss. Employ methods like bypassing certain cells during charging to focus on weaker cells.
- Monitoring the State of Health (SoH): Keeping track of the SoH provides insights into the battery’s condition and remaining lifespan. A drop in SoH indicates the need for maintenance or replacement.
6 Primary Types of Lithium Ion Batteries
Lithium ion batteries, a cornerstone of modern technology, come in several types, each with unique characteristics and applications. The diversity in lithium ion batteries stems from the various active materials used in their construction, influencing their performance, longevity, and suitability for different uses.
Key Types of Lithium Ion Batteries
- Lithium Iron Phosphate (LFP): Known for their durability and safety, LFP batteries use phosphate in the cathode and a carbon electrode in the anode. These batteries are known for their long life cycle and good thermal stability. They are ideal for replacing lead-acid deep-cycle batteries due to their nominal voltage and stability.
- Lithium Cobalt Oxide (LCO): These batteries are notable for their high specific energy but are limited in high-load situations. They were commonly used in portable electronics like phones and laptops but have seen a decline in popularity due to cost and safety concerns.
- Lithium Manganese Oxide (LMO): LMO batteries, used in tools and some hybrid vehicles, offer quick charging and high specific power. They stand out for their improved thermal stability and versatility in different applications.
- Lithium Nickel Manganese Cobalt Oxide (NMC): Combining nickel, manganese, and cobalt, NMC batteries balance stability with high energy density. They are frequently used in power tools and electric vehicles.
- Lithium Nickel Cobalt Aluminium Oxide (NCA): Offering high specific energy and a respectable lifespan, NCA batteries are a popular choice in the electric vehicle industry, particularly for high-performance models like Tesla.
- Lithium Titanate (LTO): Unique for using lithium titanate instead of graphite in the anode, LTO batteries are exceptionally safe and fast-charging. They are used in various applications, including electric vehicles and energy storage, despite their lower energy density and higher cost.
LiFePO4 Batteries: Leading the Charge in Lithium-Ion Technology
Lithium Iron Phosphate (LiFePO4) batteries are one of many types of lithium-ion batteries, each defined by different cathode materials. Other common types include Lithium Cobalt Oxide (LCO), Lithium Manganese Oxide (LMO), Lithium Nickel Cobalt Aluminum Oxide (NCA), Lithium Nickel Manganese Cobalt Oxide (NMC), and Lithium Titanate (LTO). Each has unique strengths and weaknesses, making them suitable for specific applications. (Learn more about Best LiFePO4 Battery)
Energy Density Comparison
LiFePO4 batteries boast one of the highest specific power ratings among lithium-ion batteries, meaning they can deliver large amounts of current efficiently. However, they have a lower specific energy, indicating less energy storage capacity per unit weight compared to other types. While this may not be a concern for many applications, it can be limiting in scenarios requiring high energy density, such as in battery electric vehicles.
Battery Life Cycles
LiFePO4 batteries excel in longevity, with lifespans starting at 2,000 full discharge cycles and potentially exceeding 5,000 cycles. This extended lifespan, only second to Lithium Titanate, offers significant advantages in terms of cost-effectiveness and environmental impact.
Discharge Rates
LiFePO4 batteries commonly feature a 1C continuous discharge rate, with the potential to exceed this under certain conditions. This capability makes them suitable for high-power applications that require current spikes at startup.
Operating Temperatures
With a high thermal runaway threshold of approximately 270 degrees Celsius, LiFePO4 batteries can operate safely under higher temperatures compared to other lithium-ion types. This characteristic, combined with robust Battery Management Systems (BMS), significantly reduces the risk of thermal runaway events.
Safety Advantages
Among all lithium-ion batteries, LiFePO4 batteries are known for their stability and safety, making them a preferred choice for both consumer and industrial applications. Their safe chemistry, alongside Lithium Titanate, is ideal for applications requiring a high degree of safety and reliability.
LiFePO4 vs. Other Lithium Ion Batteries
While LiFePO4 batteries may not be optimal for small, wearable devices due to lower energy density, they excel in larger applications like solar energy systems, RVs, golf carts, and electric motorcycles. They surpass other lithium-ion batteries in cycle life, safety, and depth of discharge capabilities.
Cycle Life and Safety
LiFePO4 batteries can achieve over 3,000-5,000 cycles, with the ability to reach 100% depth of discharge without over-discharging risks. This longevity, combined with their inherent safety, makes them the safest lithium battery type available, surpassing lithium-ion and other battery types in safety metrics.
Environmental and Efficiency Advantages
Eco-friendly and rechargeable, LiFePO4 batteries outperform lead-acid batteries in terms of efficiency, life span, and environmental impact. They charge faster, have a lower self-discharge rate, and maintain consistent power even below 50% battery life, all with no maintenance requirements.
Size and Weight Benefits
LiFePO4 batteries are significantly lighter than other lithium and lead-acid batteries, enhancing fuel efficiency and maneuverability in vehicles. Their compact size also frees up space for additional applications.
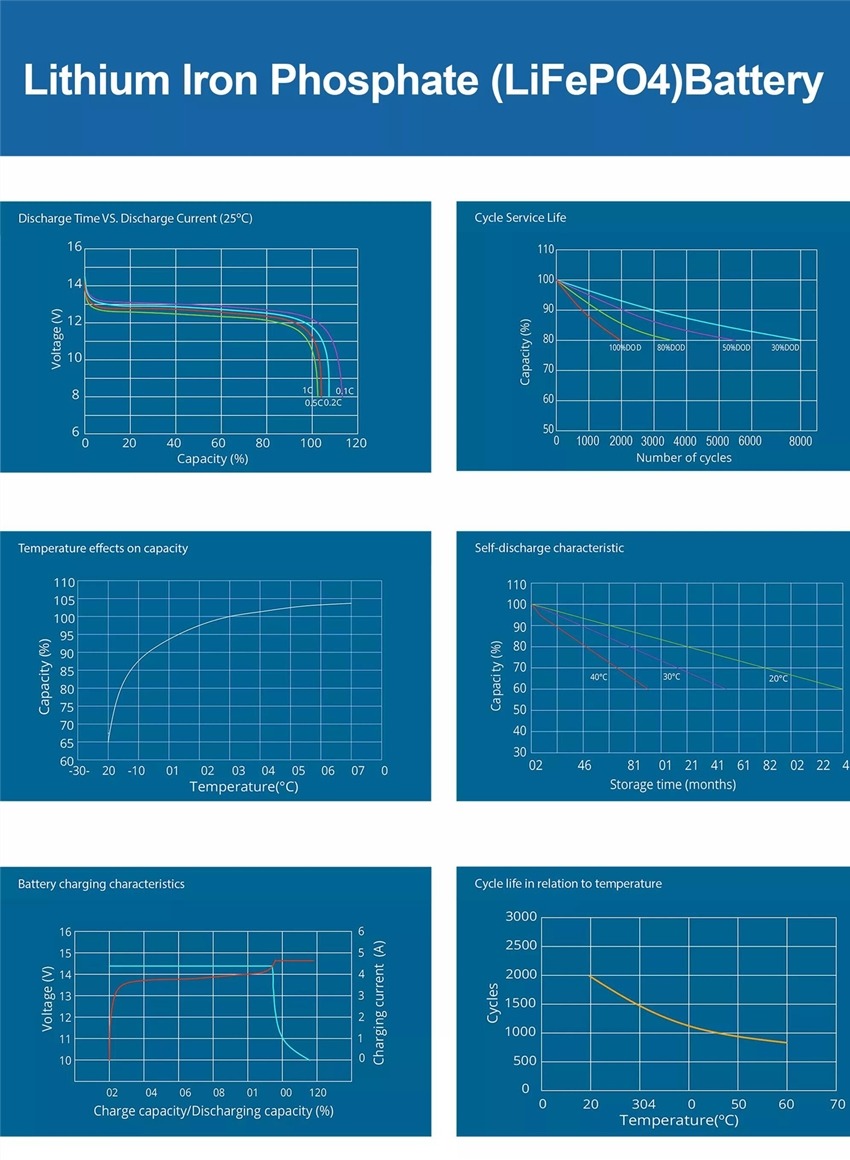
Lithium Iron Phosphate Battery Vs Lead acid
Lithium iron phosphate battery:
- Durability: Lithium iron phosphate battery has strong durability, slow consumption, more than 2000 charging and discharging times, and no memory, and the general life span is 5-8 years.
- Discharge rate: Lithium iron phosphate battery can be discharged with high current, suitable for solar street lights, electric cars, electric bicycles, etc.
- In terms of volume and quality: Lithium batteries are relatively small in size.
- Battery capacity: The capacity of lithium batteries in the same volume is larger. The lead-acid battery has a capacity of about 20 amps; the lithium battery has a capacity of 8-10 amps.
- No memory effect: Lithium iron phosphate battery can be charged and used at any time, no matter what state it is in. It does not need to be discharged before recharging.
- Nominal voltage of monomer: The nominal voltage of lithium iron phosphate battery is 3.2V.
- Environmental protection: Lithium materials do not contain any toxic and harmful substances, and are regarded as green and environmentally friendly batteries in the world. The batteries are pollution-free in production and use, and have become a hot research topic.
- Safety: Lithium iron phosphate has passed strict safety tests and will not explode even in the worst traffic accidents, showing higher safety performance.
Lead-acid batteries:
- Lead-acid batteries are generally deep-charged and discharged within 300 times, have memory, and have a lifespan of about two years. And there is liquid in the lead-acid battery, after a period of consumption, if the battery is found to be hot or the charging time becomes shorter, you need to replenish the liquid. To
- Generally, the weight of lead-acid batteries is 16-30 kg, and the volume is relatively large;
- There is a large amount of lead in lead-acid batteries. If they are not handled properly after being discarded, they will pollute the environment and cause pollution during the production process. To
- The nominal voltage of a lead-acid battery is 2V, and the voltage of an ordinary lead-acid battery is usually 12V.

Comparison Table: LiFePO4 vs. Lead Acid Battery
| Feature | LiFePO4 Battery | Lead Acid Battery |
|---|---|---|
| Energy Density | High energy density, more power per unit weight. | Lower energy density. |
| Weight | Lighter, making them more suitable for portable applications. | Heavier, less suitable for applications where weight is a concern. |
| Cycle Life | Typically 2000 – 5000 cycles or more. | Around 300 – 500 cycles. |
| Efficiency | High efficiency, around 85-95%. | Lower efficiency, about 80-85%. |
| Charge Time | Fast charging capability. | Slower charging compared to LiFePO4. |
| Operating Temperature Range | Wider operating temperature range. | More limited temperature range, performance declines in extreme temperatures. |
| Maintenance | Low maintenance, no need for regular watering. | Requires regular maintenance like watering and equalization. |
| Safety | Generally safer, more stable chemistry, lower risk of thermal runaway. | Risk of acid spills and gas emissions, requires careful handling. |
| Environmental Impact | Environmentally friendlier, contains no heavy metals. | Contains lead, a toxic heavy metal, requires careful disposal. |
| Lifespan | Longer lifespan, can last up to 5 years or more. | Shorter lifespan, typically 3-5 years. |
| Cost Over Life Cycle | Initially more expensive but cost-effective over its life cycle. | Cheaper initially but less cost-effective in the long run due to shorter lifespan and maintenance. |
MANLY LiFePO4 Battery vs Other Brands LiFePO4 Battery
MANLY LiFePO4 Batteries stand out in the competitive battery market with their robust combination of innovation, quality, and versatility. Backed by over 13 years of expertise, these batteries, hailing from China’s technological hubs, offer unparalleled customization, catering to diverse applications from solar energy storage to advanced robotics. Unlike standard LiFePO4 batteries, MANLY Batteries boast a remarkable 98% energy efficiency rate, enhanced safety features, and global certifications like UN38.3, IEC62133, UL, and CE. Their commitment to durability and user experience is further exemplified by features like Bluetooth connectivity and intuitive displays. This focus on consumer-centric innovation and superior performance makes MANLY Batteries a leading choice for those seeking reliable, high-quality battery solutions, distinctly setting them apart from other brands in the market.
| Feature | MANLY LiFePO4 Battery | Other Brands LiFePO4 Battery |
|---|---|---|
| Key Competitive Advantage |
|
|
| Customization Options | Offers customization in voltage, capacity, current, dimensions, aesthetics, etc. | Customization varies by manufacturer; not all offer extensive options. |
| Certifications | Boasts UN38.3, IEC62133, UL, CE, among others. | Certification depends on the manufacturer; common ones include CE, UL. |
| Warranty | 10-year warranty. | Warranty periods vary, typically around 1-5 years. |
| Protection Features | Includes short circuit, overcharge/discharge, balancing circuits, overvoltage/overcurrent, and safety against explosion or ignition. | Standard protections include overcharge/discharge, short circuit, and temperature control. |
| Operating Conditions | Operates in -20°C to 75°C. (Advised not to charge below 0°C) | Operating temperature ranges vary, typically -10°C to 60°C. |
| Efficiency Rate | Energy efficiency rate of 98%. | Efficiency rates vary, typically around 85-95%. |
| Enhanced Features | Optional BMS, Bluetooth connectivity and battery level display. | Additional features vary by model and manufacturer. |
| Waterproof | Up to IP67 waterproof rating (depends on the customer’s waterproof requirements for the product) | Not all productions have waterproof |
| Lifespan | Lifespan of 8000+ cycles , typically 10-20 years (Longevity relies on MANLY’s expert R&D team) | Lifespan varies, typically 5-10 years depending on usage and maintenance. |
Unlocking the Potential of Lithium Batteries Across Industries
Let’s delve into the various industries where lithium batteries shine as the preferred power source:
Cold Weather Battery Guide: Top Picks for 2023
Choosing The Right Battery for Electric Scooter
Best Lithium Marine Battery: Guide to Selecting in 2023
The 6 Best Lithium Golf Cart Batteries
1. Battery capacity
Table of Contents
Generally the capacity of the battery is determined by the amount of active material in the battery, usually expressed in milliampere-hour mAh or Ah. For example, 1000 mAh can be discharged for 1 h with a current of 1 A.
1.1 Understanding Battery Capacity: Rated vs. Actual vs. Theoretical
Battery capacity can be categorized into actual capacity, theoretical capacity, and rated capacity, based on different conditions.
The capacity that a battery provides when discharged at a particular discharge rate at 25°C down to its terminal voltage is defined as the battery’s capacity during design and production. This is termed the rated capacity for a given discharge rate RH.
Battery capacity is typically measured in AH (Ampere-hours). Another method of measurement is in terms of CELL (per unit plate) in watts (W/CELL).
- For Ah (Ampere-hours) calculations, you take the discharge current (constant current) I and multiply it by the discharge time (in hours) T. For instance, a 7AH battery discharged continuously at 0.35A can last for approximately 20 hours.
- The standard charging time is 15 hours, and the charging current is 1/10 of the battery’s capacity. Fast charging may reduce the battery’s lifespan.
Battery capacity refers to the size of the stored electrical charge in a battery. The unit for battery capacity is “mAh”, known in Chinese as milliampere-hours. For larger capacity batteries, such as lead-acid batteries, “Ah” (Ampere-hours) is generally used for convenience, where 1Ah = 1000mAh. If a battery has a rated capacity of 1300mAh, and you discharge the battery with a current of 130mA, the battery can operate for about 10 hours (1300mAh/130mA = 10h). If the discharge current is 1300mA, then the operational time drops to around 1 hour. These calculations assume ideal conditions, and actual device operation may vary based on components, like an LCD screen or flash in a digital camera, which can cause large variations in current. Hence, the actual operational time for a device powered by the battery can only be approximated, usually based on real-world experience.
1.2 Unit of Capacity
Typically, battery capacity is measured in ampere-hours (Ah), and this is determined for a specific battery in mind. For instance, the question might be: What’s the capacity of this smartphone battery? Or, what’s the capacity of this electric scooter battery? These queries are unique to each battery. When the battery voltage is already defined and one isn’t considering the real-time voltage, merely stating the ampere-hours can represent the battery’s capacity.
However, when dealing with batteries of different voltages, one can’t simply rely on ampere-hours to denote capacity. Take, for instance, a MANLY 12V 20AH battery and a MANLY 15V 20AH battery. Even though both have 20AH, when powering a device with the same load, the device will work just fine, but the duration of operation will differ. Hence, the standard capacity should be measured in terms of power.
To illustrate further, consider a device that can support both 12V and 24V. If powered by a MANLY 12V 20AH battery, it can last for one hour. However, if two such batteries are connected in series, resulting in 24V 20AH, the ampere-hours remain unchanged, but the operational time doubles. In this context, the capacity should be viewed in terms of the power the battery can hold, not just the ampere-hours.
Power (W) = Power (P) * Time (T) = Current (I) * Voltage (U) * Time (T).
This approach to discussing battery capacity is more meaningful. One must be realistic and factual; otherwise, you might end up with the illogical claim that a smartphone battery has a larger capacity than an electric scooter battery, which is clearly unscientific.
2. Nominal voltage
The potential difference between the positive and negative electrodes of the battery is called the nominal voltage of the battery. The nominal voltage is determined by the electrode potential of the plate material and the concentration of the internal electrolyte. The discharge diagram of lithium battery is parabolic, with 4.3V dropping to 3.7V and 3.7V dropping to 3.0V, both of which change rapidly. Only the discharge time of about 3.7V is the longest, accounting for almost 3/4 of the time, so the nominal voltage of the lithium battery refers to the voltage that maintains the longest discharge time.
- Ternary Lithium Battery
The nominal voltage of a ternary lithium cell is 3.6V, with an operating voltage range between 2.5V and 4.2V. For a battery pack, you multiply the voltage by the number of cells in series. For instance, for a 10-series ternary lithium battery pack, the nominal voltage stands at 36V, and the working voltage range spans from 25V to 42V.
- Lithium Iron Phosphate Battery
The nominal voltage of a lithium iron phosphate cell is 3.2V, with an operational voltage range of 2.0V to 3.65V. Similarly, for the corresponding battery pack, you multiply by the number of cells in series. For example, a 15-series lithium iron phosphate battery pack has a nominal voltage of 48V, with a working voltage range of 30V to 54.75V.
So, what are the implications of a low voltage for lithium batteries?
It’s recommended that lithium batteries be stored long-term with about 70% of their charge. If not in use for 3 to 6 months, it’s advisable to cycle through one full charge and discharge. This benefits the overall lifespan of the battery pack.
If stored for extended periods without use and at very low voltages, the materials in the lithium battery can be adversely affected. Their chemical reactivity might deteriorate, which in turn impacts the battery pack’s lifespan.
Lithium-ion batteries operate at voltages ranging from 2.5V to 4.2V. When the voltage drops below 2.5V, the battery discharge terminates, and due to the closing of the discharge circuit, the current loss of the internal protection circuit drops to its lowest. However, in real-world applications, due to variations in internal materials, the discharge termination voltage can range from 2.5V to 3.0V. When the voltage exceeds 4.2V, the charging circuit is terminated to ensure the battery’s safety.
3. Charge termination voltage
When the rechargeable battery is fully charged, the active material on the electrode plate has reached a saturated state, and the battery voltage will not rise when the battery continues to be charged. The voltage at this time is called the end-of-charge voltage. The ternary lithium battery is 4.2V, and the lithium iron phosphate battery is 3.65V.

4. Discharge termination voltage
The end-of-discharge voltage refers to the lowest voltage allowed when the battery is discharged. The discharge termination voltage is related to the discharge rate.
5. Internal resistance of the battery
The internal resistance of the battery is determined by the resistance of the electrode plate and the resistance of the ion flow. During the charging and discharging process, the resistance of the image engine and the electrode plate is unchanged, but the resistance of the ion flow will increase or decrease with the concentration of the electrolyte and the charged ions. And change. When the OCV voltage of a lithium battery decreases, the impedance will increase. Therefore, when charging at low power (less than 3V), pre-charge (trickle charging) must be carried out first to prevent too much current from causing excessive heat generation of the battery.
Composition of Lithium Battery Internal Resistance
Ohmic resistance mainly arises from the electrode materials, electrolyte, separator resistance, as well as the contact resistance of current collectors and tab connections. It’s related to the battery’s size, structure, and connection methods.
Polarization resistance, which emerges instantly when current is applied, represents the cumulative tendency of various barriers inside the battery preventing charged ions from reaching their destinations. This resistance can be further categorized into electrochemical polarization and concentration polarization.
Currently, the standout 18650 lithium battery has an internal resistance of around 12 milliohms, while typical ones hover between 13 to 15 milliohms. Given that impedance can affect the battery’s performance, generally speaking, 50 milliohms is deemed normal. Between 50 to 100 milliohms, the battery remains functional, but performance starts to degrade. When exceeding 100 milliohms, parallel use is necessary, and anything above 200 milliohms is virtually unusable.
Impacts of Lithium Battery Internal Resistance
All factors that impede the movement of lithium ions and electrons from one pole to another within the lithium battery contribute to its internal resistance. Ideally, the lower the internal resistance, the better. A higher internal resistance leads to increased thermal losses, preventing high current discharge. Moreover, a high internal resistance means the battery heats up during use. Elevated temperatures can cause the battery’s discharge operating voltage to drop and its discharge duration to shorten, severely affecting battery performance and lifespan. In extreme cases, this can even pose a risk of spontaneous combustion.
6. Self-discharge rate
It refers to the percentage of the total capacity that is automatically lost when the battery is not in use for a period of time. Generally, the self-discharge rate of lithium-ion batteries at room temperature is 5%-8%.
6.1 How Does the Discharge Rate Affect Battery Capacity?
The discharge rate directly impacts a battery’s effective capacity. Specifically, a higher discharge rate can decrease the available capacity, as the battery might not be able to maintain its maximum rated capacity during rapid discharges. Thus, when evaluating a battery’s usable capacity, the discharge rate must be taken into account.
6.2 What is the Discharge Rate of Electric Bicycle Batteries?
The discharge rate of electric bicycle batteries can vary based on the specific battery chemistry and design. Electric bikes typically employ lithium-ion batteries due to their high energy density and performance. These batteries generally demonstrate a discharge rate ranging from 1C to 4C or even higher. To illustrate, a 1Ah battery with a 10C discharge rate can deliver a continuous discharge current of 10 amps, whereas a 4C rate would allow for a continuous discharge current of 40 amps.
6.3 What is Considered a High Discharge Rate for Lithium Batteries?
For lithium batteries, a discharge rate typically considered “high” starts at 1C and above. However, it’s important to note that what’s deemed as a high specific discharge rate may vary based on the battery’s design, chemical composition, and intended application.
6.4 What is a Good Discharge Rate for Batteries?
An optimal C-rate for a battery hinges on the specific demands of its application. Typically, a discharge rate that facilitates efficient power transfer without overly stressing the battery is regarded as favorable. It’s recommended to consult the manufacturer’s specifications and guidelines to ascertain the best discharge rate for a particular battery.
6.5 How Do You Calculate the C-rate?
C-rate (C) = Charging or discharging current in amperes (A) / Battery’s rated capacity (Ah)
Let’s delve into an example involving a 100Ah lithium battery:
1C represents a discharge current of 100 amps, meaning the battery can provide a continuous discharge of 100 amps for one hour. In simpler terms, it can handle a load current of 100 amps for 60 minutes.
If we boost the C-rate to 2C, the discharge current becomes 200 amps. This signifies that the battery can now furnish a discharge current of 200 amps, but for a reduced duration. At a 2C rate, the battery can sustain a load current of 200 amps for 30 minutes or half an hour.
On the other hand, reducing the C-rate to 0.5C results in a discharge current of 50 amps. At a 0.5C rate, the battery can deliver a discharge current of 50 amps, thereby prolonging the discharge period. Under these conditions, the battery can support a load current of 50 amps for 2 hours or 120 minutes.
When assessing the performance and capacity of lithium batteries, the C-rate stands out as a crucial factor since it determines both the available discharge current and the corresponding discharge duration.
The reason why lithium batteries need protection is determined by their own characteristics. Since the material of the lithium battery itself determines that it cannot be overcharged, overdischarged, overcurrent, short circuited, and ultra-high temperature charge and discharge, the lithium battery components of the lithium battery will always appear with an exquisite protection board.
Ordinary lithium battery protection boards usually include control ICs, MOS switches, resistors, capacitors and auxiliary devices FUSE, PTC, NTC, ID, memory, etc. Among them, the control IC controls the MOS switch to turn on under all normal conditions to make the cell and the external circuit conduct, and when the cell voltage or loop current exceeds the specified value, it immediately controls the MOS switch to turn off to protect the cell’s Safety.
When the protection board is normal, Vdd is high, Vss and VM are low, DO and CO are high. When any parameter of Vdd, Vss, VM is changed, the level of DO or CO will be Changes.
1. Overcharge detection voltage: Under normal conditions, Vdd gradually rises to the voltage between VDD and VSS when the CO terminal changes from high level to low level.
2. Overcharge release voltage: In the charging state, Vdd gradually decreases to the voltage between VDD and VSS when the CO terminal changes from low level to high level.
3. Overdischarge detection voltage: Under normal conditions, Vdd gradually decreases to the voltage between VDD and VSS when the DO terminal changes from high level to low level.
4. Overdischarge release voltage: In the overdischarge state, Vdd gradually rises to the voltage between VDD and VSS when the DO terminal changes from low level to high level.
5. Overcurrent 1 detection voltage: Under normal conditions, VM gradually rises to the voltage between VM and VSS when DO changes from high level to low level.
6. Overcurrent 2 detection voltage: In the normal state, VM rises from OV to the voltage between VM and VSS when the DO terminal changes from high to low at a speed of 1ms or more and 4ms or less.
7. Load short-circuit detection voltage: Under normal conditions, VM starts from OV and rises to the voltage between VM and VSS when the DO terminal changes from high level to low level at a speed of 1μS or more and 50μS or less.
8. Charger detection voltage: In the over-discharge state, VM gradually decreases with OV to the voltage between VM and VSS when DO changes from low level to high level.
9. Current consumption during normal operation: Under normal conditions, the current (IDD) flowing through the VDD terminal is the current consumption during normal operation.
10. Over-discharge current consumption: In the discharging state, the current (IDD) flowing through the VDD terminal is the over-current discharge current consumption.